MARIE CURIE, HENRI BECQUEREL, WILHELM RÖNTGEN
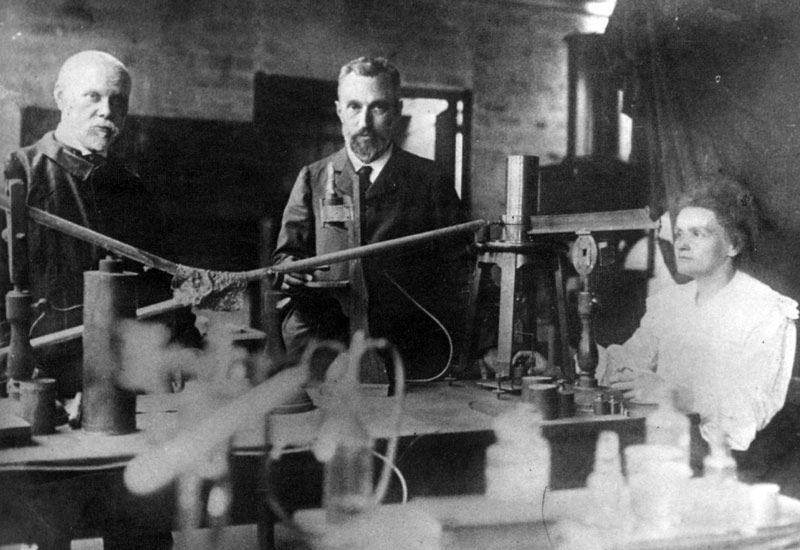
The modern understanding of ionizing radiation got its start in 1895 with Wilhelm Röntgen. In the process of conducting various experiments in applying currents to different vacuum tubes, he discovered that, despite covering one in a screen to block light, there seemed to be rays penetrating through to react with a barium solution on a screen he’d placed nearby. After several experiments, including taking the first photo (of his wife’s hand and skeletal structure) with the new rays, he named them “X-Rays” temporarily as a designation of something unknown, and the name stuck.
“It seemed at first a new kind of invisible light. It was clearly something new, something unrecorded...” - WILHELM RÖNTGEN
This discovery was followed in 1896 by Henri Becquerel’s discovery that uranium salts gave off similar rays naturally. Though originally thinking that the rays were given off by phosphorescent uranium salts after prolonged exposure to the sun, he eventually abandoned this hypothesis. Through further experimentation including non-phosphorescent uranium, he instead came to recognize that it was the material itself that gave off the rays.
Although it was Henri Becquerel that discovered the phenomenon, it was his doctoral student, Marie Curie, who named it: radioactivity. She would go on to do much more pioneering work with radioactive materials, including the discovery of additional radioactive elements: thorium, polonium, and radium. She was awarded the Nobel Prize twice, once alongside Henri Becquerel and her husband Pierre in Physics for their work with radioactivity, and again years later in Chemistry for her discovery of radium and polonium. She also conducted pioneering work in radiology, developing and deploying mobile X-ray machines for the battlefields of World War I.
“We must not forget that when radium was discovered no one knew that it would prove useful in hospitals. The work was one of pure science. And this is a proof that scientific work must not be considered from the point of view of the direct usefulness of it. It must be done for itself, for the beauty of science, and then there is always the chance that a scientific discovery may become like the radium, a benefit for mankind. ” - MARIE CURIE
She died in 1934 of aplastic anemia, probably developed from extended exposure to various radioactive materials, the dangers of which were only really understood long after most of her exposure had occurred. In fact, her papers (and even her cookbook) are still highly radioactive and many are considered unsafe to handle, stored in shielded boxes and requiring protective equipment to safely review.
RADIUM WATCH DIAL PAINTERS
One of the first major events to highlight the dangers of ionizing radiation was the case of the “Radium Girls,” workers whose job was painting watch dials with radium. Though there was enough suspicion of the effects of ionizing radiation for the management of the company to take precautions, they offered none to the actual workers painting the watch dials. Many of them would lick their brushes to properly shape them. Since the human body treats radium as calcium, it was then deposited in the bones and led to radiation sickness. It is unknown how many died from radiation exposure.

After five of the workers sued the company (United States Radium), and the ensuing publicity, the health risks of radiation exposure were brought to public attention. The public interest and the availability of a large sample set (up to 4000 people were employed at dial painters over the years) led to the first long-term study of radiation exposure. Finally ending in 1993, it provided a wealth of information on the long-term effect of radiation exposure. The case also provoked drastic changes in both the fields of workplace safety & liability, and the field of Health Physics, dealing with the health effects and safety issues involved in working with radioactive materials.
THE MANHATTAN PROJECT & THE COLD WAR
The Manhattan Project, the crash study undertaken during World War II to develop the first atomic bomb, led directly the second long term study of the effects of long-term radiation exposure, namely the study of the survivors of the bombs at Hiroshima and Nagasaki. The bombings, which killed more than 150,000 between them (with some estimates putting the total at closer to 245,000 or more), also left more than 600,000 survivors (hibakusha, literally “explosion affected people”), many who have been studied in the years since. Among the findings was that there does not appear to have been an increase of birth defects in those that survived the blasts. There have been, however, about 1900 cancer deaths that can be directly attributable to the bombings.

Since the creation and detonation of the atomic bombs ushered in the “Atomic Age,” much has changed in our understanding and implementation of radiation and radioactive material. Throughout the Cold War, there was experimentation on both sides into the properties and uses of radioactive material in various test reactors and related sites, looking to harness both the strategically valuable offensive power of radioactive material for nuclear weapons and the potentially valuable uses in other fields such as medicine,
Radiation is the process by which energy is emitted as either particles or waves. Broadly, it can take the form of sound, heat, or light. However, most people generally use it to refer to radiation from electromagnetic waves, ranging from radio waves, though the visible light spectrum, and up through to gamma waves.

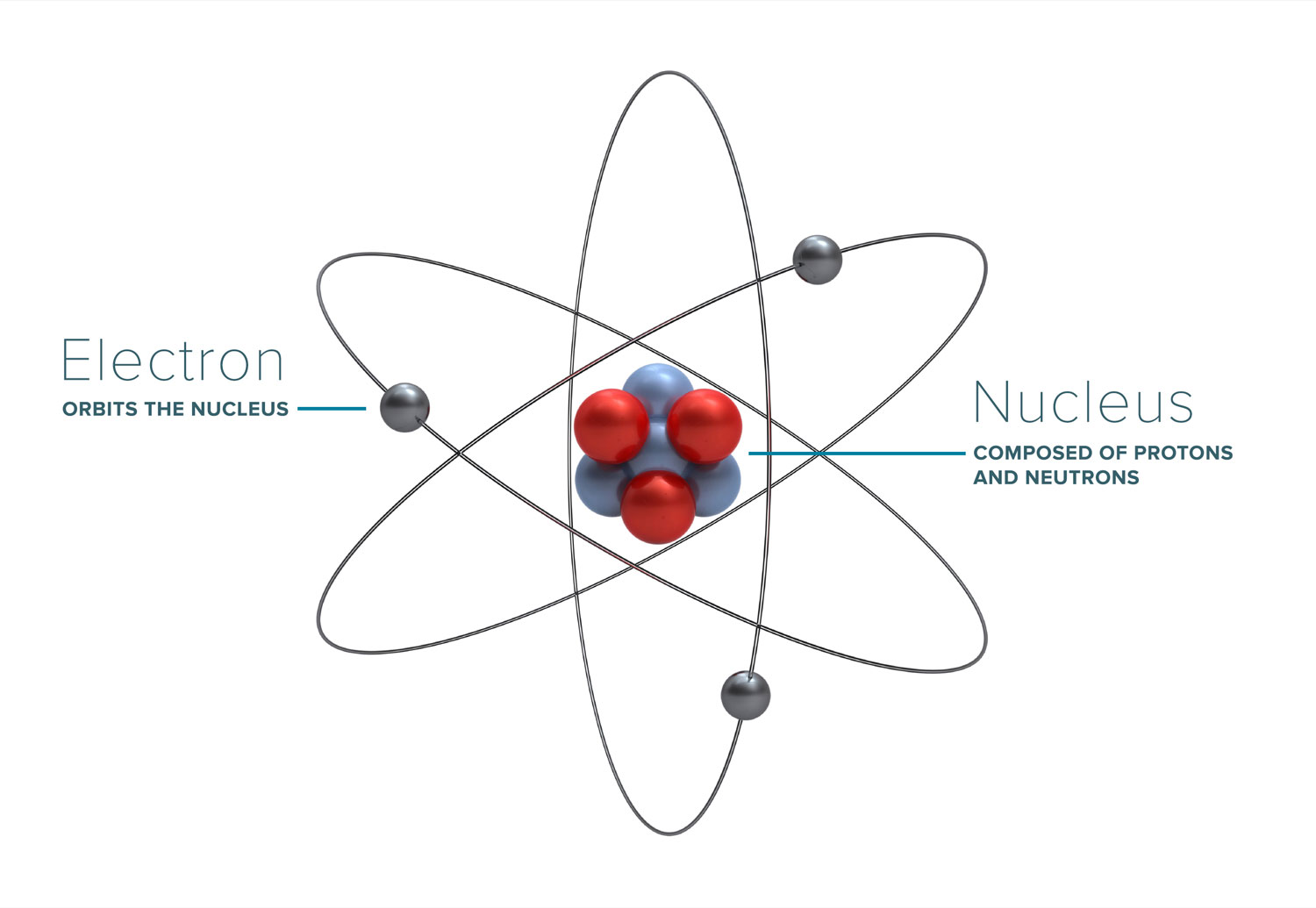
ATOMS AND THEIR PARTS
Most of the discussion about radiation, how it works, and what its effects are boil down to the interaction of radiation with atoms (and molecules) that it comes into contact with. Atoms form the basic building blocks of all matter. They consist of a nucleus, made of positively-charged protons (and sometimes neutrally-charged neutrons), and an outer cloud of electrons, which have a negative charge. The positive charge of a single proton is equal to the negative charge of a single electron.
Protons and neutrons have a relatively large size and atomic weight, whereas electrons are extremely small and light by comparison. Due to the nature of opposite charges attracting, atoms tend to have an equal number of protons and electrons, leaving the atom as a whole having a net charge of zero. However, if the atom either loses or gains an electron, it becomes an ion, and carries a charge.
It will seek bonds with other charged particles in order to regain a neutral balance, potentially leading to new molecules being formed.
IONIZING VS NON-IONIZING RADIATION
Radiation is generally classified ionizing or non-ionizing, based on whether it has enough energy to knock electrons off atoms that it interacts with, as well as being able to do lower-energy damage such as breaking chemical bonds in molecules. Ionizing radiation, which is caused by unstable atoms giving off energy to reach a more stable state, is more of a health threat to humans because it involves changing the basic makeup of atoms in cells, and more specifically the DNA molecules inside of cells. It does, of course, take a very strong dose of radiation to substantially damage a cell’s structure, as there can be trillions of atoms in a single cell.
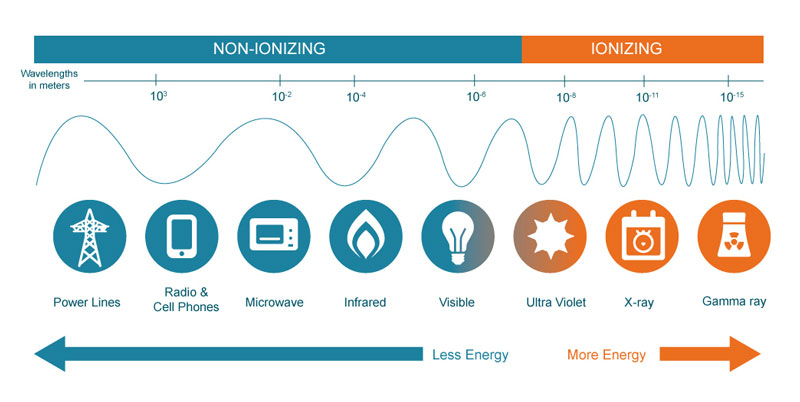
Most non-ionizing radiation, such as radio and microwave energy, is considered harmful only to the extent of the amount of heat energy it transfers to whatever it hits. This is, in fact, the way that microwaves cook food. UV light is unique in that while it is non-ionizing, it does have the capacity to cause harmful effects similar to what ionizing radiation can create, such as an increased risk of cancer due to damage to DNA molecules.
HOW IS RADIATION MEASURED?
The radioactivity of a substance, or how “active” it is radioactively, is measured in either curies (Ci) or Becquerel’s (Bq). Both are measures of the number of decays per second, or how often an atom in a given sample will undergo radioactive decay and give off a particle or photon of radiation. The curie (1 Ci equals about 37,000,000,000 decays per second) is named after Marie and Pierre Curie, and is equal to roughly the activity of one gram of radium, which they studied. The Becquerel is the SI unit for radioactivity. One Bq equals one decay per second. The Bq is the SI unit, though the curie remains widely used throughout the US in both government and industry.
TYPES OF of RADIATION
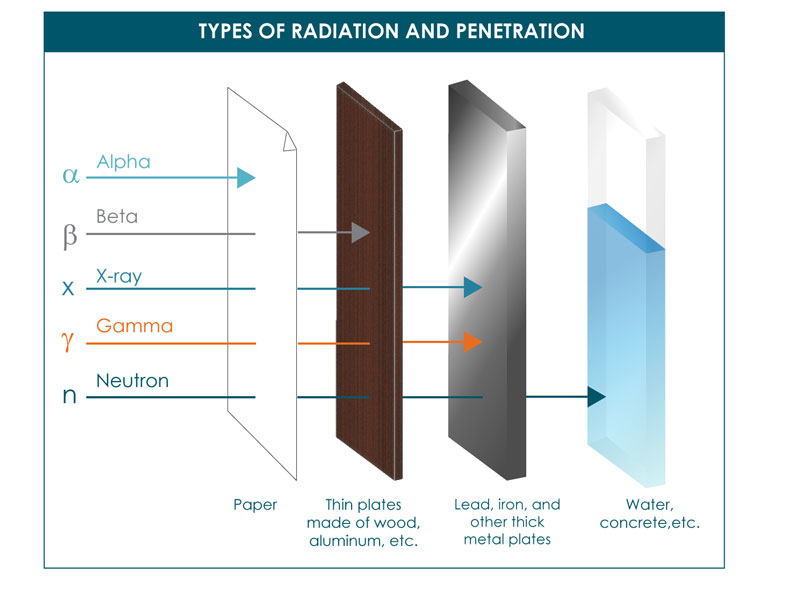
Ionizing radiation takes a few forms: Alpha, beta, and neutron particles, and gamma and X-rays. All types are caused by unstable atoms, which have either an excess of energy or mass (or both). In order to reach a stable state, they must release that extra energy or mass in the form of radiation.
ALPHA RADIATION
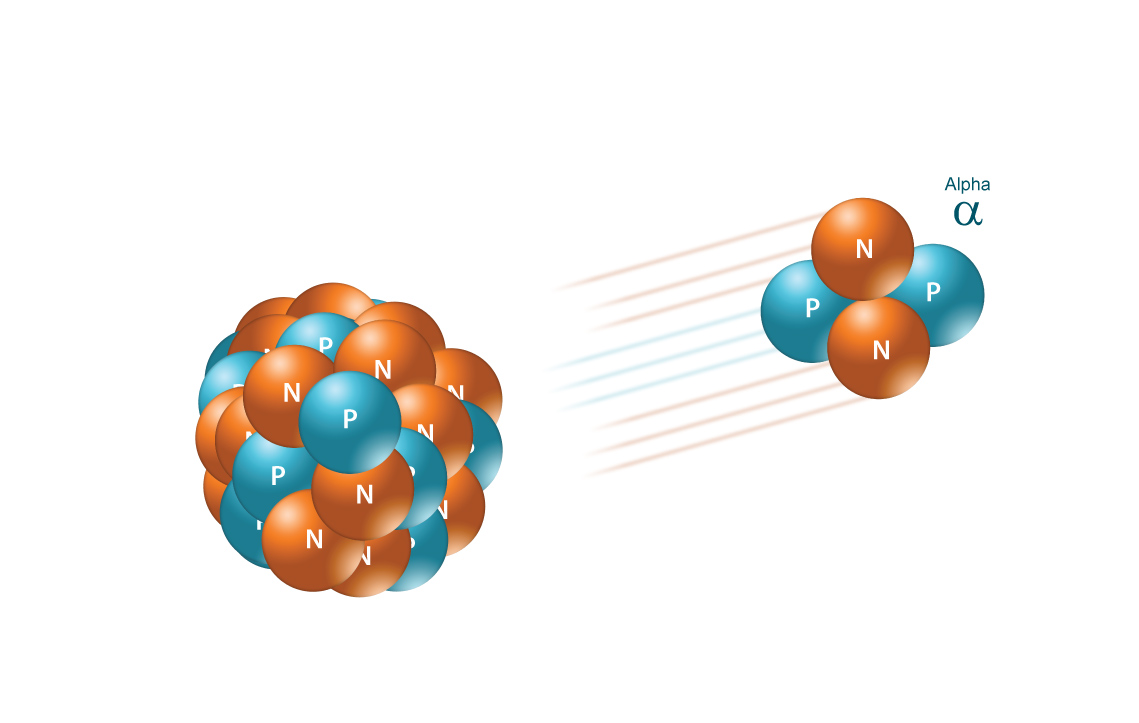
Alpha radiation occurs when an atom undergoes radioactive decay, giving off a particle (called an alpha particle) consisting of two protons and two neutrons (essentially the nucleus of a helium-4 atom), changing the originating atom to one of an element with an atomic number 2 less and atomic weight 4 less than it started with. Due to their charge and mass, alpha particles interact strongly with matter, and only travel a few centimeters in air. Alpha particles are unable to penetrate the outer layer of dead skin cells, but are capable, if an alpha emitting substance is ingested in food or air, of causing serious cell damage. Alexander Litvinenko is a famous example. He was poisoned by polonium-210, an alpha emitter, in his tea.
BETA RADIATION
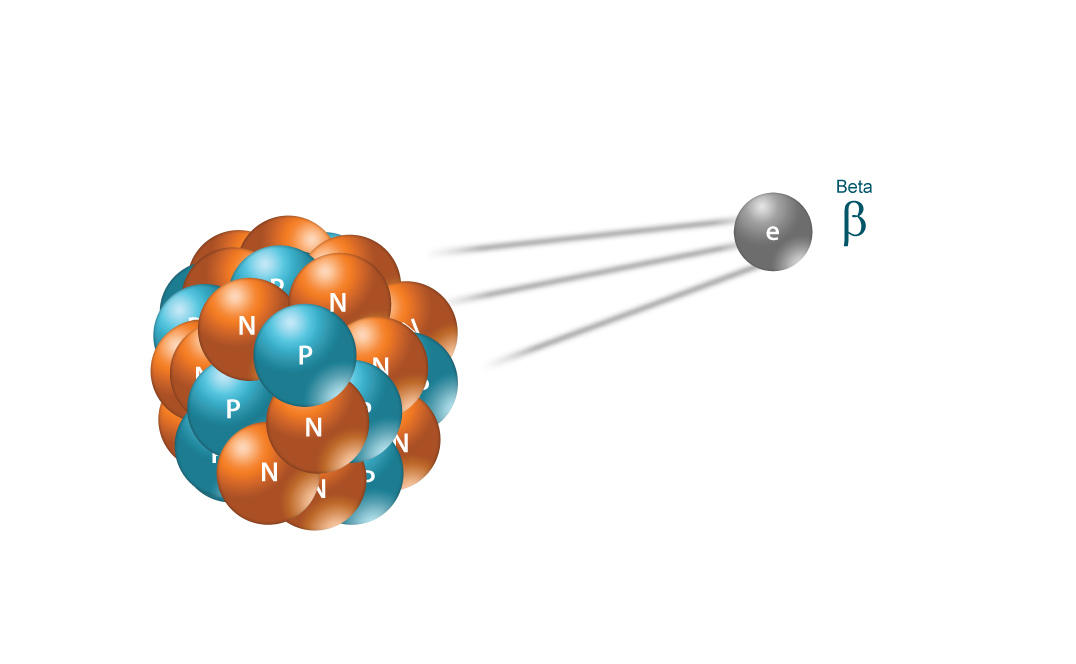
Beta radiation takes the form of either an electron or a positron (a particle with the size and mass of an electron, but with a positive charge) being emitted from an atom. Due to the smaller mass, it is able to travel further in air, up to a few meters, and can be stopped by a thick piece of plastic, or even a stack of paper. It can penetrate skin a few centimeters, posing somewhat of an external health risk. However, the main threat is still primarily from internal emission from ingested material.
GAMMA RADIATION
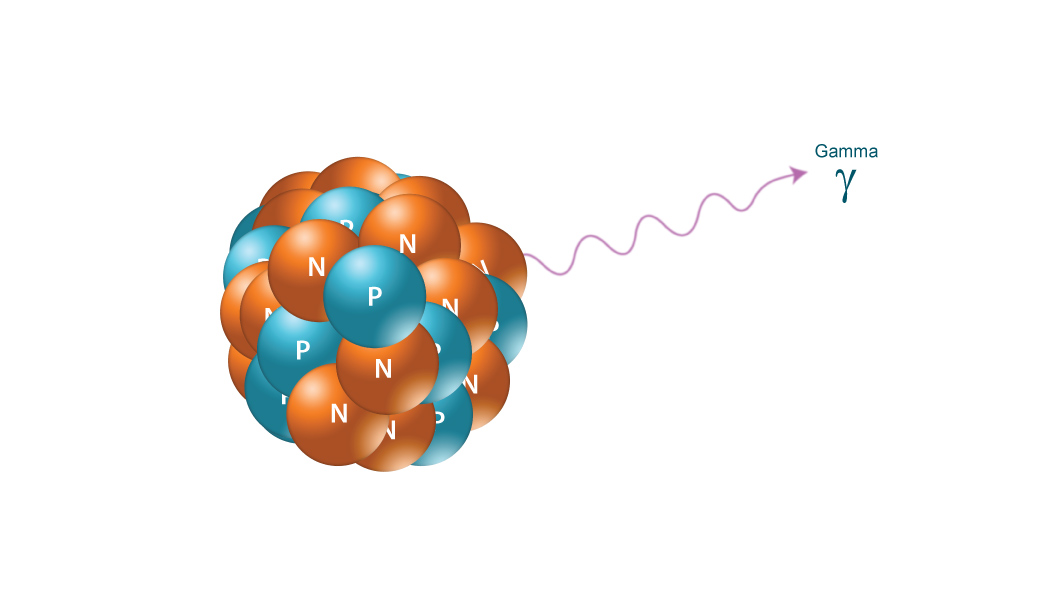
Gamma radiation, unlike alpha or beta, does not consist of any particles, instead consisting of a photon of energy being emitted from an unstable nucleus. Having no mass or charge, gamma radiation can travel much farther through air than alpha or beta, losing (on average) half its energy for every 500 feet. Gamma waves can be stopped by a thick or dense enough layer material, with high atomic number materials such as lead or depleted uranium being the most effective form of shielding.
X-RAYS
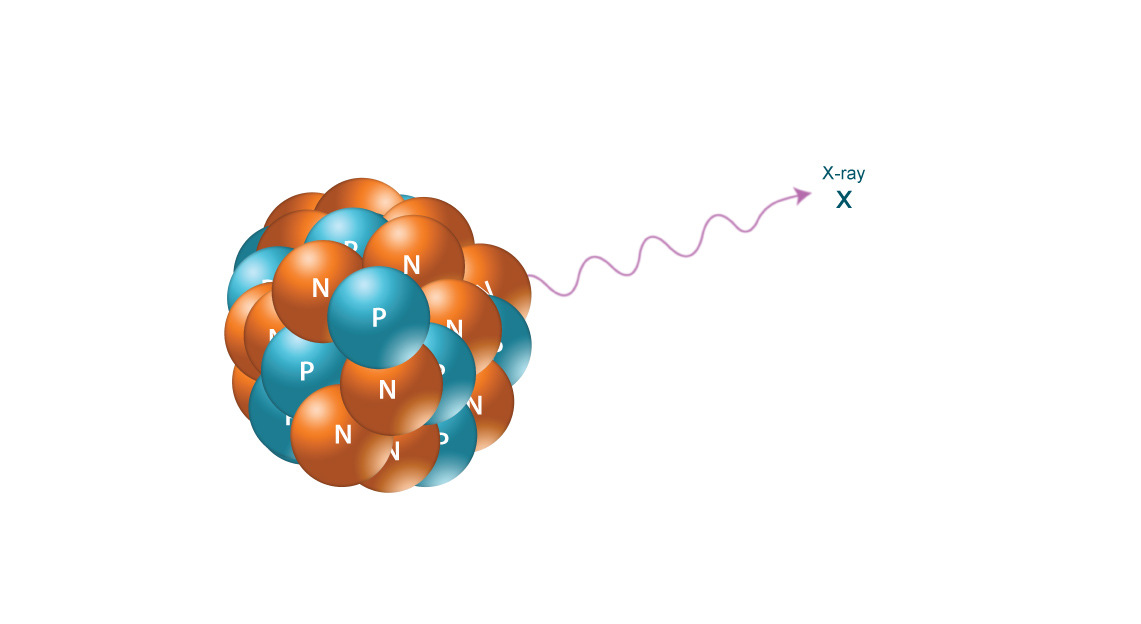
X-rays are similar to gamma radiation, with the primary difference being that they originate from the electron cloud. This is generally caused by energy changes in an electron, such as moving from a higher energy level to a lower one, causing the excess energy to be released. X-Rays are longer-wavelength and (usually) lower energy than gamma radiation, as well.
NEUTRON RADIATION
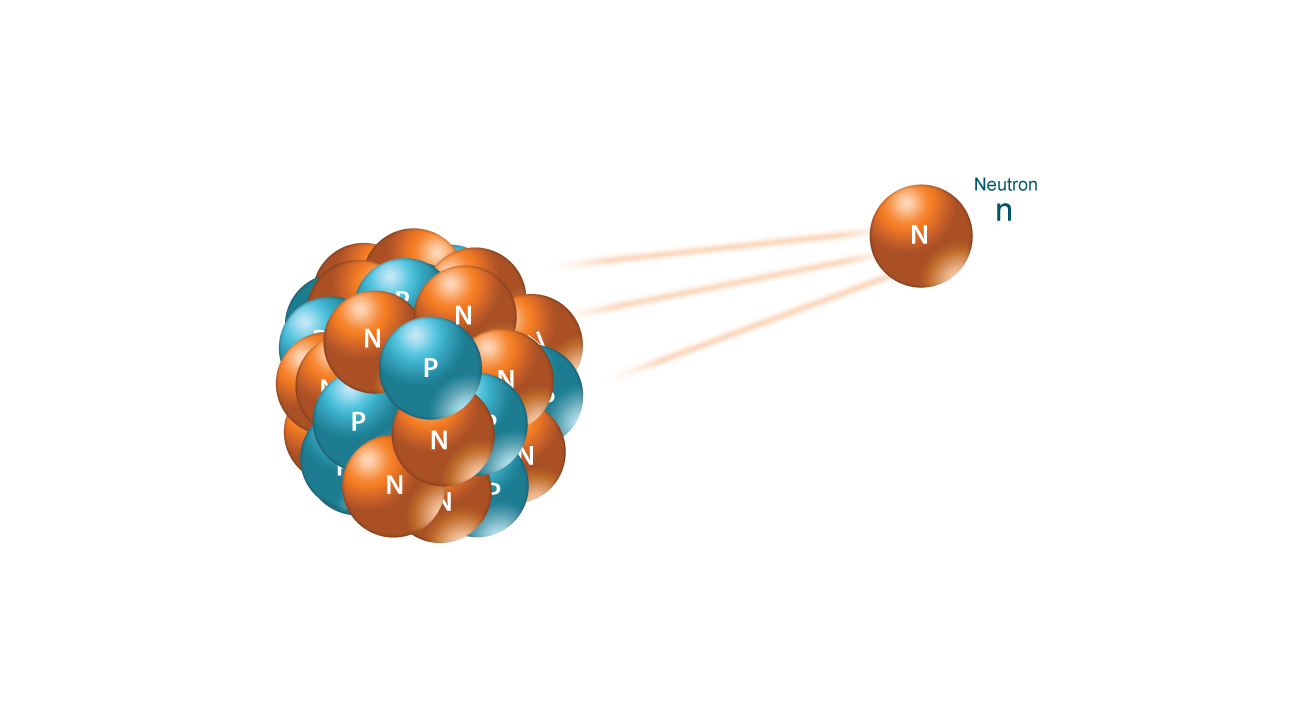
Lastly, Neutron radiation consists of a free neutron, usually emitted as a result of spontaneous or induced nuclear fission. Able to travel hundreds or even thousands of meters in air, they are however able to be effectively stopped if blocked by a hydrogen-rich material, such as concrete or water. Not typically able to ionize an atom directly due to their lack of a charge, neutrons most commonly are indirectly ionizing, in that they are absorbed into a stable atom, thereby making it unstable and more likely to emit off ionizing radiation of another type. Neutrons are, in fact, the only type of radiation that is able to turn other materials radioactive.
NATURALLY OCCURRING RADIATION & NORM

Radioactive material is fairly common in nature, and generally pretty harmless in that state. It’s important to have an understanding of how much of the radioactive material a person comes across in a given day is from the natural environment around them.
BANANAS
One of the most commonly encountered sources of natural radiation is one that many people don’t suspect. Bananas, being naturally very high in potassium, consequently have a higher than usual amount of potassium-40, a radioactive isotope. In fact, the term “banana equivalent dose” has come into pretty common use as a reference point for communicating radiation exposure.

Other common foods that contain elevated levels of radioactive elements include carrots and white potatoes, which carry slightly lower levels of potassium-40. Lima beans have almost 50% more potassium-40 than bananas, and also very small amounts of radon-224. The food with the highest concentration of radioactive elements, in this case radium, is the Brazil nut. In all these foods, however, the levels are extremely low and not considered harmful, and almost none of the radioactive material consumed while eating any of them is retained in the body. The fat content of the Brazil nuts would pose a health risk long before the radiation in them, for example.
THE SUN
Powered by a continuous nuclear reaction, it comes as little surprise that the sun gives off quite a bit of radiation. There’s also a good bit of cosmic radiation origination from sources outside the solar system. Luckily for life on earth, however, most of that energy is intercepted and absorbed by the Earth’s magnetosphere and the ozone layer.
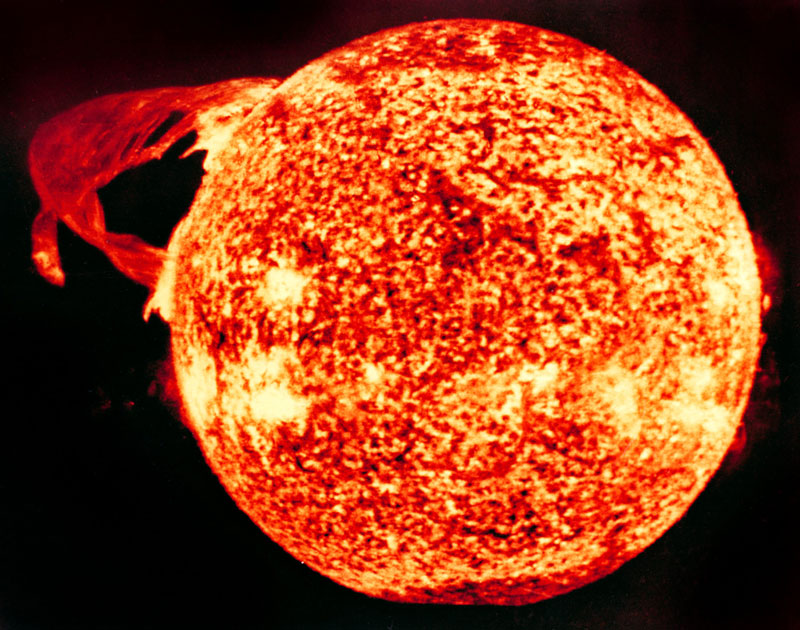
Cosmic radiation does, however, make up a small percentage (about 13%) of the total annual background radiation a person is exposed to over the course of a year. This exposure rate is slightly increased by living at higher altitudes, and even more so by air travel. Flight crews on long-distance, high-altitude flights tend to accumulate about 30% more annual radiation exposure than the average person.
THE GROUND BENEATH OUR FEET
(Radium, uranium, from Mining/Oil)
The other major source of naturally occurring radiation is from minerals and materials buried in the earth. Most common are potassium-40, uranium-238, and thorium-232, which all have fairly long half-lives. Additionally, there are small quantities of shorter-lived materials, such as radium-226, which is a decay product of U-238, and radon-222, which is a product of Ra-226. Radon, being a gas, can become a problem in some houses and other buildings, seeping in usually through cracks in solid foundations, and accumulating in rooms with poor ventilation. In the United States, the Appalachian Mountains region of southeast Pennsylvania, along with parts of Iowa, have the most problems with radon concentration, due to a larger deposit of uranium in the bedrock of those areas.

Another area where terrestrial radiation can become something of an issue is in the oil and natural gas mining industries, where it is referred to as Naturally Occurring Radioactive Material, or NORM. Often dredged up in pipes in process of mining exploration and production, radioactive elements like radon and radium will bind with molecules in fluid being used, bringing it up to the surface and potentially contaminating pipelines, tanks, and other equipment.
INSIDE YOU!
It may come as a surprise to some people, but since the human body is made up of many of the same atoms and elements that are found in the rest of the terrestrial environment, a certain percentage of those atoms are radioactive. Most common are Carbon-14, since life is carbon-based, and Potassium-40, since Potassium forms an important part of DNA molecules.

The presence of radioactive Carbon-14 in living organisms actually forms the basis of radiocarbon dating of organic material, due to the fact that levels of C-14 in plants and animals match ambient levels in the atmosphere at the time of their death. Afterwards, however, the C-14 decays (with a half life of around 5730 years) and by measuring the remaining amount, and comparing it with known or calculated amounts at different times. Through this method, the age of a sample up to around 45,000 years can be fairly accurately determined.
MAN-MADE SOURCES OF RADIAT

Outside of naturally occurring sources of radiation, there are man-made processes that produce radioactive material that the average person may come into contact with on a regular basis. The levels are still fairly low, with annual exposure to the public due to artificial sources being roughly equal to the dose received from natural sources.
GRANITE COUNTERTOPS, FIESTAWARE
One of the more commonly found, if relatively weak and low-impact, sources of artificially generated radiation exposure is simply from the use of naturally occurring elements in man-made items. Granite contains trace amounts of uranium, and some samples can end up with a high enough concentration of uranium to be measureable with detection equipment.

Other places that radioactive material may be found in a household setting are in some varieties of vintage Fiestaware (or any red glazed pottery from the 40s-60s) that used uranium in order to achieve the bright colors in the glaze. Modern products use artificial dyes to achieve the same colors, of course.
NUCLEAR POWER
Nuclear power is perhaps the first thing that springs to mind when you ask someone about man-made sources of radiation. Using fission reactions in uranium to turn water into steam to power giant turbine generators, nuclear power plants generate tremendous amounts of electricity. Much of Europe gets electricity from nuclear power plants, as do many sections of the US.
Nuclear power plants are tightly regulated, with tight limits on both radiation exposure to workers and the public. The annual average dose to a member of the public from nuclear power plants is roughly equal to the amount generated internally by the decay of radioactive materials naturally found in the body. In fact, due to the presence of radioactive Uranium and Thorium in the fly ash from coal burning, coal-burning power plants give off more radiation into the environment, typically, than a nuclear power plant.
TERRORISM, DISASTER, WAR
The detonation of the atomic bombs at Hiroshima and Nagasaki, along with the nuclear weapons tests of the 50s and 60s, deposited a certain amount of radioactive fallout into the atmosphere. The Limited Test Ban treaty of 1963 mostly put an end to this, though both France and China continued testing into 1974 and 1980, respectively, as neither country is a signatory of the treaty. The 1986 explosion at the Chernobyl nuclear power plant in Ukraine also contributed to global levels of atmospheric radioactive material, with a much greater impact in the immediate areas due to elements such as Strontium-90 and Iodine-131, leading to an increase in the incidence of thyroid cancer.

Despite all the possible health issues arising from the increase in atmospheric radiation due to fallout from nuclear testing and accidents, there has been one area where it has presented a surprising benefit. Authorities in charge of preventing the poaching of elephants for their ivory have discovered that it’s possible to tell if the ivory is recently poached, or “antique” based on the presence of these elements, since they are absorbed by the bones of living animals. So ivory that is purported to be hundreds of years old, which contains elevated levels of strontium-90, for example, will be able to be seized as illegal.
RADIATION VS. CONTAMINATION
common misconception is the idea that exposure to radiation in turn makes someone radioactive. This is, usually, not the case. It’s important, then, to understand the differences between radiation, and radioactivity.
WHAT DOES IT MEAN TO BE RADIOACTIVE?
An atom is said to be “radioactive” if it is unstable due the excess of either energy or mass, and is therefore likely to decay at some point and give off radiation. A substance or material is said to be “radioactive” if it is made up of or contains a large quantity of a radioactive material. These radioactive materials, such as bananas, the uranium glaze in vintage fiestaware, or NORM generated in the process of natural gas exploration, give off radiation over time as the radioactive atoms in them decay.

Over time, as the number of unstable atoms decreases, the material becomes less radioactive. This time is measured by the “half life” of different radioactive elements. This is the amount of time it takes for half of the atoms in a given sample to decay and give off radiation. For example, carbon-14 has a half-life of 5730 years, so after that amount of time, a quantity of 100 atoms of C-14 would have turned into 50 C-14 atoms and 50 Nitrogen-14 atoms. Iridium-11, a radioactive isotope used in medicine as a tracer, has a half-life of 2.8 hours; whereas another isotope of iridium at the other end of the scale, iridium-115 has a half-life of 441 trillion years. It’s commonly held that a sample of radioactive material will be completely decayed after 7 half lives, though after that time there would still be about 0.78% left, which with a large enough starting sample would still be significant. For smaller samples like those typically used in medicine, though, it’s a good rule of thumb.
WHAT IS CONTAMINATION?
Put simply, radioactive contamination is just radioactive material somewhere it shouldn’t be. This could be anything from nuclear fallout from a dirty bomb (the whole purpose of which would be to disperse radioactive contaminant), to a lab worker splashing some of a radioactive solution on his pants and taking them home. The most common source of contamination is from mistakes or accidents in the production of radionuclides, like those used in the medical field.

Contamination on or in a surface can be either “fixed” or “removable.” An example of fixed contamination, or contamination that isn’t able to be removed, would be in metal recycling: If a batch of recycled metal included something with radioactive material in it, the final product would have that radioactive material mixed in and permanently part of it. Removable contamination is, of course, removable, such as a loose powder or something that can be cleaned and safely disposed of. Disposal of radioactive waste can consist of reprocessing it for commercial use, though in some cases where this isn’t possible the best solution is burying it in concrete, rock, as this helps prevent the spread of the contamination any further.
DOES BEING EXPOSED TO RADIATION MAKE ME RADIOACTIVE?
Exposure to radiation does not immediately make a person radioactive. The only type of radiation that is capable of directly causing other material to become radioactive is neutron radiation, which is generally only found inside nuclear reactors or in a nuclear detonation. Anyone in those conditions is, put plainly, going to have bigger problems.
However, the ingestion of radioactive material does have the potential of making a person radioactive, at least on a temporary basis. This is the principle behind the medical use of many radioactive materials, as it aids in imaging, diagnosis, and other areas. Between the short half-lives of the elements involved and the body’s natural means of disposing of many radioactive elements, a person’s individual radioactivity is usually short-lived. However, certain types of contamination, depending on the isotopes involved and the availability of treatment, can become more permanently deposited in a person’s organs or bones.
USES OF RADIATION
Outside of nuclear power and nuclear weaponry, there remains a wide array of ways in which radioactive material and the radiation it gives off remain useful in the daily lives of people all over the world.
SMOKE DETECTORS
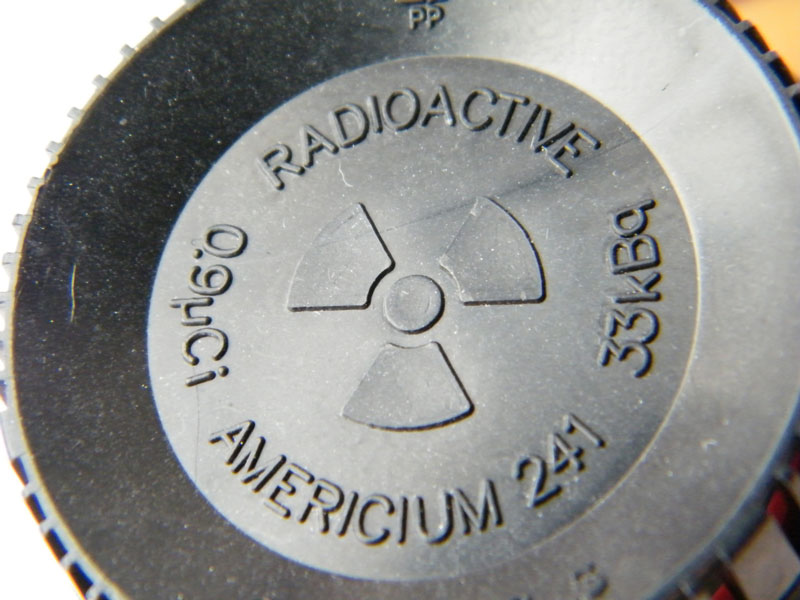
Some smoke detectors also use radioactive elements as part of their detection mechanism, usually americium-241, which use the ionizing radiation of the alpha particles to cause and then measure changes in the ionization of the air immediately around the detector. A change due to smoke in the air will cause the alarm to sound.
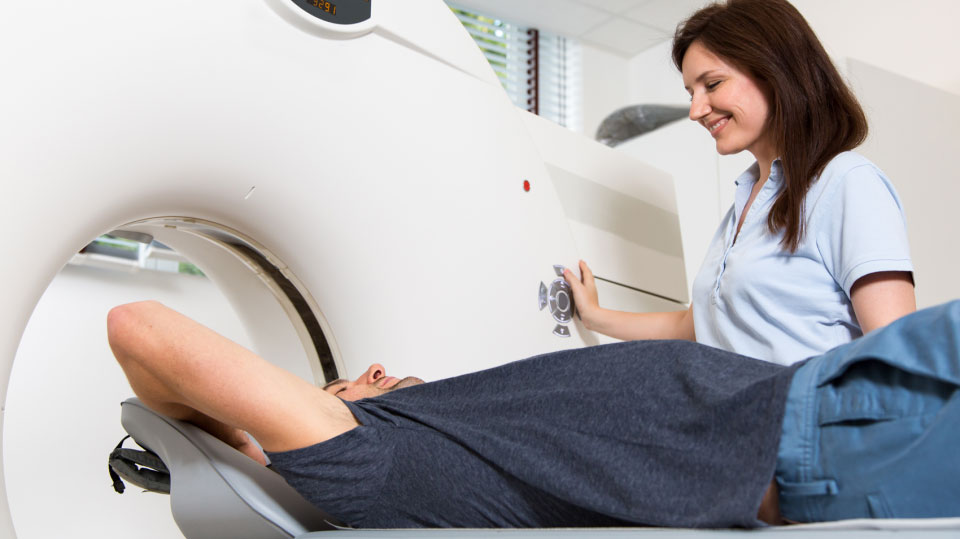
MEDICINE
Hospitals use radiation in a wide range of ways. X-Ray, CT, and PET machines use X-ray (X-ray and CT) and Gamma radiation (PET) to produce detailed images of the human body, which provide valuable diagnostic information for doctors and their patients. Radionuclides are also used to directly treat illnesses, such as radioactive iodine, which is taken up almost exclusively by the thyroid, to treat cancer or hyperthyroidism. Radioactive tracers and dyes are also used to be able to accurately map a specific area or system, such as in a cardiac stress test, which may use a radioactive isotope like Technetium-99 to identify areas of the heart and surrounding arteries with diminished blood flow.
RADIOGRAPHY
Essentially high-powered versions of the types of X-Ray machines used in medicine, industrial radiography cameras use X-rays or even gamma sources (such as Iridium-192, Cobalt-60, or Cesium-137) to examine hard to reach or hard to see places. This is frequently used to examine welds for defects or irregularities, or examining other materials to locate structural anomalies or internal components.

Industrial radiography is also very useful for secure, non-invasive scanning at security checkpoints, such as airports, where x-ray baggage scanners are in routine use. Larger versions of the same machines are often used to examine shipping containers all over the world.
FOOD SAFETY

Food irradiation is the process of using radioactive sources to sterilize foodstuffs. The radiation works by killing bacteria and viruses, or eliminating their ability to reproduce by severely damaging their DNA or RNA. Since neutron radiation is not used, the remaining food doesn’t become radioactive itself, leaving it safe to eat. This method is also used to sterilize food packaging, medical devices, and manufacturing parts.
HOW DOES RADIATION AFFECT ME?
Probably the most important question a person has about radiation is what exposure to it will do to them. It is an invisible, odorless energy that has a lot of mystery about it, so fears and speculation tend to be pretty common. In the wake of events such as Fukushima, additionally, media coverage tends to present a lot of factual information, but without some of the context that would help make it clearer to the viewer.

HOW IS RADIATION EXPOSURE MEASURED?
Radiation exposure is measured primarily in Rem (in the US) and the Sievert (SI unit), and is a measure of the radioactive dose absorbed relative to its possible health effects on the body. This is called the “equivalent dose,” and is weighted to account for the fact that the same amount of time in an alpha radiation field, for example, would have different long-term effects as the same time in a gamma field of equal strength.

Rem is broken down further into millirem (mrem) and microrem (µrem), which are the levels that are going to usually be talked about. Another common usage is in talking about dose rate, given in rem/hr or mrem/hr, which is a useful measurement of the field strength in an area, designating how quickly someone will reach a given dose level.
ACUTE VS. CRONIC EXPOSURE
A big factor in determining the effects of radiation exposure is whether it is “acute” or “chronic.” Acute exposure is a dose of radiation received all at once. Examples include doses involved in cancer therapy. The immediate concern with acute exposure would be Acute Radiation Syndrome, which would occur at about 150 to 350 rem whole body exposure. As a reference point, a chest CT scan, which is one of the highest-dose “common” sources of exposure, delivers about 1 rem of dose. A passenger on a flight across the continental US would receive 4 mrem, or 0.004 rem.

Chronic exposure, on the other hand, is low levels of exposure over a long period of time. Examples could be exposure from high levels of radon in a basement, or someone living at high altitudes. Residents of the Andes Mountains, for example, receive around 200 mrem from cosmic radiation annually, about 6 times more than the average. Chronic radiation exposure, at low levels, presents a much reduced health risk, as it is of low quantities over long periods of time, allowing the body to repair any damage made to the cells. The main health concern with chronic exposure is an increased risk of cancer, as seen by the increase in thyroid cancers found in Belarus since the Chernobyl disaster, with between 4000 and 6000 cases being directly attributable to the increased radiation exposure, with 15 deaths reported.
HEALTH EFFECTS OF EXPOSURE
Radiation exposure can have varying effects, depending on the dose received, and what the exposure was. Certain elements, when the exposure is internal, will deposit in various organs or bones. Radioactive Iodine tends to seek out the thyroid (making it useful in cancer treatment) whereas Strontium-90, which tends to seek out bone and bone marrow, and can lead to bone cancer and leukemia.
For acute exposures, the first physical effects can be seen at around 25-50 rem, and manifests as a drop in a person’s white blood cells. Acute Radiation Syndrome occurs at 150-350 rem, presenting nausea, fatigue, hair loss, and skin reddening. The LD 30/50, which is the point where 50 percent of the people exposed will die within 30 days without medical care, is between 460 and 600 rem. At 1000 rem, 100% of those exposed will die within 60 days.
These are all very high levels compared to typical exposure. Eating a banana, for example, is 0.01 millirem of exposure. The average person receives about 30 mrem of dose from the decay of potassium-40 in their own bodies every year. The maximum external dose received by members of the public after the Three Mile Island accident was about 100 mrem, or 25% of typical annual background radiation.
RADIATION IN FOOD & WATER
A source of concern for many people is the prospect of radioactive contamination of their food or water. This can occur from radioactive contaminants being ingested by animals that are then used for food, such as Strontium-90 found in cow’s milk after Chernobyl, or taken up by plants through their root systems. This is how Brazil nuts become radioactive, due to their root system taking up radium from the soil.

In situations where surface contamination of food is a concern, detection with handheld survey equipment can be useful. However, in cases such as contaminated meat or seafood, or contamination of water or other liquids such as milk, it generally requires the examination of samples using laboratory equipment to determine the presence and quantity of any contaminants.
GOVERNMENT REGULATION OF RADIATION & EXPOSURE
Given the risks and the public concerns involved, there are many government agencies and regulations dealing with radioactive materials and exposures. Governing everything from production of medical isotopes to monitoring for the transportation of terrorist material, these agencies help keep the danger to the public from radiation as low as possible.

DIFFERENT AGENCIES AND WHAT THEY REGULATE
(NRC, DHS, FTA, ETC)
In the US, there are many agencies at work. For the nuclear power industry, the primary regulatory agency is the NRC, or Nuclear Regulatory Commission. They monitor existing plants and their emissions, they provide licensing for new plants, and oversee the handling and disposal of nuclear waste material. One of the key aspects of what the NRC does is dictate the limits of exposure to the public, along with other guidelines and limitations on the nuclear industry to ensure that safety standards are met.

Other agencies involved include the Department of Homeland Security, which coordinates with various state agencies and organizations to provide radiation security for facilities and events. The Federal Transit Administration also is involved, dictating the means by which radioactive material may be shipped and how it must be labeled and placarded.
OCCUPATIONAL VS NON OCCUPATIONAL LIMITS
For the nuclear industry, the NRC, among other things, dictates exposure limits to both workers dealing with radioactive material, called the occupationally exposed, and the general public, or non-occupationally exposed. For an occupationally exposed worker, such as someone at a nuclear power plant or in nuclear medicine at a hospital (if they’re licensed by the NRC), the limit is 5 rem a year. Surprisingly, while most nuclear power workers never receive anywhere close to that amount, some workers in the medical field, such as those working with X-ray fluoroscopy machines, are amongst the highest occupationally exposed workers. Pregnant women who are occupationally exposed may choose to (but are not required to) declare their pregnancy and receive lower dose limits throughout the term of the pregnancy.
For members of the public, the annual limit from the NRC (which is matched by the EPA for areas not covered by NRC guidelines) is 100 mrem. Licensed facilities have to have programs in place to limit exposure, and be able to demonstrate that procedures are in place that members of the public would not be exceeding those levels.
HAZMAT TEAMS & OTHER AGENCIES THAT PERFORM LOCAL SURVEYS
Some states and municipalities have departments that are equipped to deal with radioactive material. Either as part of a local fire department, a stand-alone hazmat team, or a larger state emergency management agency, these groups are equipped to perform radiological surveys of areas suspected of having radioactive contamination, be it from an automobile accident involving shipment of sources, to a suspected terrorist incident.

It’s generally recommended to contact one of these agencies if there’s a suspected issue with radioactive contamination or exposure. Given the complexity of attaining and interpreting the data, and the necessity of following procedures to ensure accuracy, it’s better to contact these groups who have the equipment and training before attempting, as an individual, to purchase and use detection equipment in such a case.
Really awesome blog. Your blog is really useful for me. Thanks for sharing this informative blog. Keep update your blog.
ReplyDeleteiso 14001 lead auditor training online
Thanks for given detail information to me. keep posting like this.
ReplyDeleteISO 17025 internal auditor training Dubai
Very Nice. This blog is very useful to me. Now I have clarified my doubts. Thanks for sharing the information. ISO 17025 Internal Auditor Training
ReplyDeleteThanks you for sharing this unique useful information content with us. Really awesome work.. ISO 22301 Lead Auditor Course Oman
ReplyDelete